by Giuliana Miglierini
Different phases of paper productions require a stable and almost neutral pH value: this represent an important factor in order to avoid sudden pH variations in the paper machine, that may cause unstable wet end conditions. Carbon dioxide is a gas that can help the stabilisation of pH in a more precise way compared to the use of strong mineral acids.
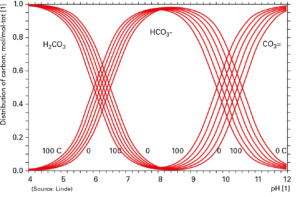
«Carbon dioxide is a very environmentally friendly chemical to use in the process», said Ann-Charlotte Jansson, Expert Pulp & Paper of Linde, presenting the possible applications of CO2 in the paper industry at the recent Tissue World Milan 2017. Carbon dioxide represents an instrument to keep under control key process parameters – such as the pH and the concentration and dissolution of calcium carbonate (CaCO3) – and helps to stabilise the entire process. «When you add CO2, you don’t add any disturbing ions to the process, or substances that may be corrosive or poisonous. It is very user friendly», said Ann-Charlotte Jansson. Carbon dioxide is a gas that causes no pH shocks at the point of addition, since the gas reacts with water forming the weak carbonic acid H2CO3 according to the reaction CO2(g) + H2O (H2CO3(aq). Carbonic acid can be present in solution at different ionisation steps according to the actual pH value (figure 1). «Depending on the pH, the relation between these carbonic species is different: at acidic pH you will have more carbonic acid, at neutral there is more bicarbonate ion, at basic pH there is carbonate ion», said Jansson
A gas for pH control
The alkaline digestion process that transforms wood into cellulosic fibres useful for tissue paper production require the use of strong pH conditions to remove lignin and other coloured substances. Extraction steps can also be coupled to oxidative reactions using oxygen or hydrogen peroxide to achieve a better breakdown and removal of lignin. The following phases of paper productions require a stable, and almost neutral pH value: this represent an important factor in order to avoid sudden pH variations in the paper machine, that may cause unstable wet end conditions. Addition of strong acids, such as sulphuric acid (H2SO4)to control pH is not advisable as the subsequent pH changes are sudden and can be difficult to control, with the result that they can affect runnability of the machine as well as the efficiency of many chemicals added to the pulp. Variations in pH are also a possible cause for breaking, since the actual value of pH – as well as the hardness of the process water determined by the concentration of free calcium ions – affect the release of the Web from solid surfaces. Alkalinity is the acid capacity, i.e. how much acid is needed to reach a certain pH level. Using CO2 for pH control preserves alkalinity, while strong acids destroy alkalinity. Depending on the final pH value, the use of CO2 means to change OH- negative ions to HCO3-, or 2OH- to CO32-. This change does not affect the measured alkalinity.
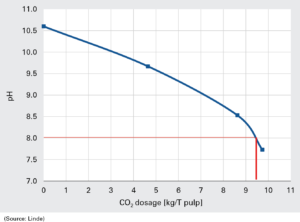
Linde presented at the Tissue World Milan its pH active control system Actico®, based only on CO2 in the gas phase, to acidify alkaline pulp or broke and to control pH in the short circulation of a paper/tissue machine. An example of this is seen in fig 2. According to Jansson, it can also be used after the alkaline boil-outs, at the wet end of paper process (figure 2).

The elevated automation of the CO2 dosage can be fine tuned according to the specific needs of each paper machine. Deinked pulp, for example, contains calcium carbonate and use of strong acid dissolves some of this CaCO3. This causes pH to rise and thus more strong acid is needed to get the pH to the desired level. Since carbon dioxide does not cause a pH shock, and since its use gives more carbonates in the system, less or no CaCO3 is dissolved.
As Ann-Charlotte Jansson explained, CO2 injectors and control system can be used to acidify the wet strength broke, that is usually characterised by high pH values. It is important to lower this value as the broke content affects also the tissue machine pH (figure 3). «pH varied depending on broke content in use. Also for people in the mill, having very high pH values is not good from the safety point of view», she said. Addition of carbon dioxide with the Actico® system to acidify the alkaline pulp allows to use as much broke as needed without deteriorating the tissue machine pH, said Jansson. Linde run at first a preparatory lab pilot, to then pass to a full scale trial where the Actico® low control unit and injector regulated the optimal CO2-dosage.
Linde’s expert also explained the importance to distinguish between pH and alkalinity, the last one corresponding to the acid capacity and defined as the sum of all different ions contributing to the carbonic acid equilibrium: [HCO3–] + 2 [CO3—] + [OH–] – [H+]. Alkalinity corresponds to the amount of acid needed to reduce the pH value to a certain level, explained Jansson. «If I have a lower alkalinity, I just need few of a strong acid and the pH suddenly drops», she said. With higher alkalinity the system has a higher buffer capacity and more acid is needed to change the pH.
Dissolution of calcium carbonate
pH stabilisation through buffering also provide the ability to increase alkalinity and prevent calcium carbonate (CaCO3) dissolution. Dissolution of calcium carbonate can cause runnability problems, formation of precipitates and deposits and a higher consumption of many chemicals. The levels of the dissolving calcium carbonate may be regulated by acting through addition of CO2 or bicarbonate ions, something that can be achieved in situ thanks to the buffering capacity of solutions containing carbon dioxide: «If pH is going down, calcium carbonate solubility increases, and you have calcium ions and bicarbonate ions in solution», said Jansson. An excess of calcium ion Ca2+ in solution is also unwanted because of the greater hardness of process water it causes, with possible formation of deposits difficult to dissolve, increased conductivity of the pulp mixture and uncontrolled formation of big CO2 bubbles. This unwanted process may also cause foaming and flotation of disturbing substances, requiring an increased dosage of defoamer or deaeretor. Excessive water hardness also results into the possible formation of holes or spots in the paper. The overall efficiency of cationic process chemicals also decreases, as well as the strength properties; the energy needs for refining increase and dewatering becomes slower.
Linde’s Adalka® process stabilizer allows to regulate and stabilize pH, according to Jansson: «Here we use a combination of CO2 and NaOH to form a sodium bicarbonate solution on site. We can vary the relation between CO2 and NaOH so to adapt it to the process and not to cause any pH shock. The bicarbonate ions can be neutralised both by acids and bases, thus they improve the stability of the process. It is used to prevent calcium carbonate dissolution», said Ann-Charlotte Jansson. The equilibrium reactions she referred to are the following:
pH stabilisation
Acid neutralisation: HCO3– + H+ (H2CO3
Base neutralisation: HCO3- + OH– (H2O + CO32-
Solubility
CaCO3(s) Ò Ca2+(aq) + CO32-(aq)
Ks.p (CaCO3) = [Ca2+ ][ CO32- + CO32- (added) ]
The Adalka® system increases buffering capacity resulting in an higher alkalinity, and it stabilizes the wet end chemistry and unit operations like beating and mixing. Increased buffering capacity also facilitates the optimization of chemical additions
According to the case study presented by Jansson, the acidification of deinked pulp, that contained solid calcium carbonate, according to the reaction CaCO3 + H+ (Ca2+ + HCO3-) resulted in 75% less dissolved Ca2+ ion than with sulphuric acid. The addition of carbon dioxide also allowed to obtain less agglomeration of hydrophobic colloids and a lower formation of spots, deposits and holes (figure 4)
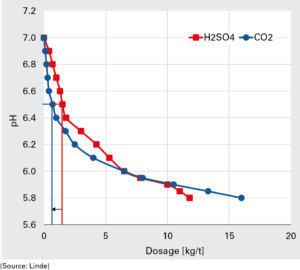
The graph shows the relation between a strong acid and CO2. According to Jansson, carbon dioxide is more efficient than strong acids at higher pH and less efficient at lower pH when neutralising “clean“ water. «But when solid CaCO3 is present, this does not hold true: the reason is that dissolution of calcium carbonate causes pH to rise and thus more acid is needed to reach the desired value. Carbon dioxide causes less or no dissolution of CaCO3 than strong acid, and thus is more efficient», said Jansson.
